What is EPR Spectroscopy?
Click here for PowerPoint version
Electron spin resonance (ESR) spectroscopy, also referred to as electron paramagnetic resonance (EPR) spectroscopy, is a versatile, nondestructive analytical technique which can be used for a variety of applications including: oxidation and reduction processes, biradicals and triplet state molecules, reaction kinetics, as well as numerous additional applications in biology, medicine and physics. However, this technique can only be applied to samples having one or more unpaired electrons.
Spectroscopy is the measurement and interpretation of the energy difference between atomic or molecular states. According to Plank’s law, electromagnetic radiation will be absorbed if:
ΔE=hν
where ΔE is the difference in energy of the two states, h is Plank’s constant and ν is the frequency of the radiation. The absorption of this energy causes a transition of an electron from the lower energy state to the higher energy state. In EPR spectroscopy the radiation used is in the gigahertz range. Unlike most traditional spectroscopy techniques, in EPR spectroscopy the frequency of the radiation is held constant while the magnetic field is varied in order to obtain an absorption spectrum.
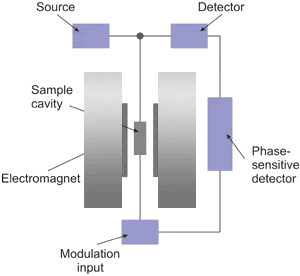
Shown above is a block diagram for a typical EPR spectrometer. The radiation source usually used is called a klystron. Klystrons are vacuum tubes known to be stable high power microwave sources which have low-noise characteristics and thus give high sensitivity. A majority of EPR spectrometers operate at approximately 9.5 GHz, which corresponds to about 32 mm. The radiation may be incident on the sample continuously (i.e., continuous wave, abbreviated cw) or pulsed. The sample is placed in a resonant cavity which admits microwaves through an iris. The cavity is located in the middle of an electromagnet and helps to amplify the weak signals from the sample. Numerous types of solid-state diodes are sensitive to microwave energy and absorption lines then be detected when the separation of the energy levels is equal or very close to the frequency of the incident microwave photons. In practice, most of the external components, such as the source and detector, are contained within a microwave bridge control. Additionally, other components, such as an attenuator, field modulator, and amplifier, are also included to enhance the performance of the instrument.
The basis of EPR spectroscopy lies in the spin of an electron and its associated magnetic moment. When an electron is placed within an applied magnetic field, Bo, the two possible spin states of the electron have different energies. This energy difference is a result of the Zeeman effect. The lower energy state occurs when the magnetic moment of the electron, μ, is aligned with the magnetic field and a higher energy state occurs where μ is aligned against the magnetic field. The two states are labeled by the projection of the electron spin, MS, on the direction of the magnetic field, where MS = -1/2 is the parallel state, and MS = +1/2 is the antiparallel state.
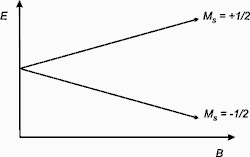
So for a molecule with one unpaired electron in a magnetic field, the energy states of the electron can be defined as:
E = gμBBoMS = ±1/2gμBBo
where g is the proportionality factor (or g-factor), μB is the Bohr magneton, Bo is the magnetic field, and MS is the electron spin quantum number. From this relationship, there are two important factors to note: the two spin states have the same energy when there is no applied magnetic field and the energy difference between the two spin states increases linearly with increasing magnetic field strength.
 |
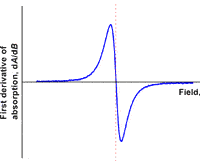 |
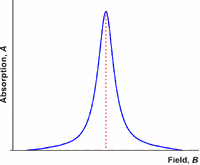
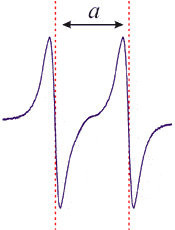
Like most spectroscopic techniques, when the radiation is absorbed, a spectrum is produced similar to the one on the left. In EPR spectrometers a phase-sensitive detector is used. This results in the absorption signal being presented as the first derivative. So the absorption maximum corresponds to the point where the spectrum passes through zero. This is the point that is used to determine the center of the signal.
As mentioned earlier, an EPR spectrum is obtained by holding the frequency of radiation constant and varying the magnetic field. Absorption occurs when the magnetic field “tunes” the two spin states so that their energy difference is equal to the radiation. This is known as the field for resonance. As spectra can be obtained at a variety of frequencies, the field for resonance does not provide unique identification of compounds. The proportionality factor, however, can yield more useful information.
![]()
For a free electron, the proportionality factor is 2.00232. For organic radicals, the value is typically quite close to that of a free electron with values ranging from 1.99-2.01. For transition metal compounds, large variations can occur due to spin-orbit coupling and zero-field splitting and results in values ranging from 1.4-3.0.
In addition to the applied magnetic field, unpaired electrons are also sensitive to their local environments. Frequently the nuclei of the atoms in a molecule or complex have a magnetic moment, which produces a local magnetic field at the electron. The resulting interaction between the electron and the nuclei is called the hyperfine interaction. Hyperfine interactions can be used to provide a great deal of information about the sample including providing information about the number and identity of nuclei in a complex as well as their distance from the unpaired electron. This interaction expands the previous equation to:
E = gμBBoMS + aMSmI
where a is the hyperfine coupling constant and mI is the nuclear spin quantum number for the neighboring nucleus.
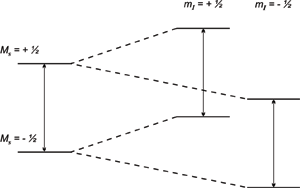
So a single nucleus with a spin ½ will split each energy level into two, as shown above, and then two transitions (or absorptions) can be observed. The energy difference between the two absorptions is equal to the hyperfine coupling constant. It is important to note that if a signal is split due to hyperfine interactions, the center of the signal (which is used to determine the proportionality factor) is the center of the splitting pattern. So for a doublet, the center would be half way between the two signals and for a triplet, the center would be the center of the middle line.
The coupling patterns that are observed in EPR spectra are determined by the same rules that apply to NMR spectra. However, in EPR spectra it is more common to see coupling to nuclei with spins greater than ½. The number of lines which result from the coupling can be determined by the formula:
2NI + 1
where N is the number of equivalent nuclei and I is the spin. It is important to note that this formula only determines the number of lines in the spectrum, not their relative intensities. Coupling to a single nucleus with spin n/2 gives (n + 1) lines each of equal intensity.
2NI + 1 = 2(1)(n/2) + 1 = n + 1 lines
For example, coupling to a single vanadium nucleus (I = 7/2) will result in a spectrum of eight lines all of equal intensity.
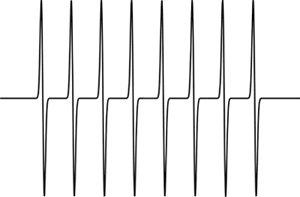 Simulated EPR spectrum showing coupling to one nucleus (/I/ = 7/2)
Simulated EPR spectrum showing coupling to one nucleus (/I/ = 7/2)
Coupling to n equivalent nuclei, each with spin ½ again gives (n + 1) lines,
2NI + 1 = 2(n)(1/2) + 1 = n + 1 lines
but, since there are multiple nuclei interacting, the relative intensities of the lines follow the binomial distribution shown below.
| # of Equivalent Nuclei | Relative Intensities |
|
1
|
1:1
|
|
2
|
1:2:1
|
|
3
|
1:3:3:1
|
|
4
|
1:4:6:4:1
|
|
5
|
1:5:10:10:5:1
|
|
6
|
1:6:15:20:15:6:1
|
An example of this is shown by the EPR spectrum of the radical anion of benzene, [C6H6•]-, in which the electron is delocalized over all six carbon atoms and therefore exhibits coupling to six equivalent hydrogen atoms. As a result, the EPR spectrum shows seven lines with relative intensities of 1:6:15:20:15:6:1. Similar distributions can be derived for n equivalent nuclei with spins greater than ½.
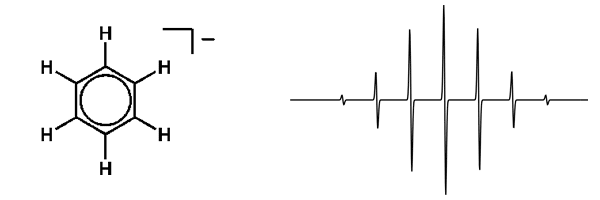 EPR Spectrum of benzene radical anion
EPR Spectrum of benzene radical anion
If an electron couples to several sets of nuclei, then the overall pattern is determined by first applying the coupling to the nearest nuclei, then splitting each of those lines by the coupling to the next nearest nuclei, and so on. An example of this can be seen in the radical anion of pyrazine. Where coupling to two equivalent 14N (I = 1) nuclei gives a quintet with the relative intensities of 1:2:3:2:1 which are further split into quintets with relative intensities of 1:4:6:4:1 by coupling to four equivalent hydrogens.
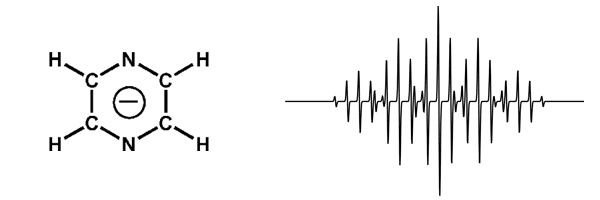 EPR Spectrum of pyrazine radical anion
EPR Spectrum of pyrazine radical anion
As NMR spectroscopy does not usually provide useful spectra for paramagnetic compounds, analysis of their EPR spectra can provide additional insight. Analysis of the coupling patterns can provide information about the number and type of nuclei coupled to the electrons. The magnitude of a can indicate the extent to which the unpaired electrons are delocalized and g-factors can show whether unpaired electrons are based on transition metal atoms or on the adjacent ligands.

