Green fluorescent protein
Understanding fundamental photophysics of the GFP choromophore through experiment and theory
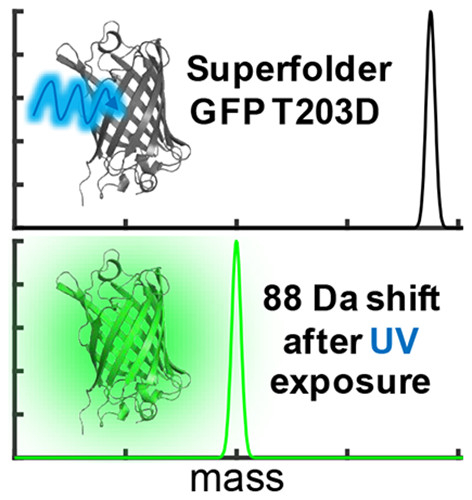
It is impossible to overstate the importance of GFP in modern biophysical and biomedical research. Although a number of GFP constructs for imaging biological details within live cells have been demonstrated, there are significant gaps in our fundamental understanding of useful chromophore properties that impede more sophisticated applications of GFP in biological research. One key area in which this is most problematic is explaining excited state electron and/or proton transfers that cause chemical reactions within the protein. Our new photoactivatable GFP variant (PA-sfGFP) provides critical insight into these important problems. We are conducting a multidisciplinary investigation into the mechanisms of photoinduced electron transfer and proton transfer in GFP that incorporates spectroscopic tools developed in our laboratory with molecular biology, synthetic chemistry, and theoretical modeling. At all points, experimental and theoretical investigations are closely coupled to fully understand the physical mechanism of the observed phenomena.
Energy transfer in superfolder green fluorescent protein
Green fluorescent proteins (GFP) have a variety of applications in biological imaging and biosensing. All of the applications rely on the photophysical and photochemical properties of the GFP and its chromophore (Chro). Photoactivatable GFP (PA-GFP) are particularly useful in these applications. However, because photoactivation mechanisms are not well understood, rational design of new PA-GFP with specific chromophore properties remains challenging. We have developed several new, closely related PA-GFPs and are mapping their excited state energy landscape in order to understand how changes in the electrostatic environment around the photoactivatable chromophore affect the mechanism of activation and the resulting photophysical properties of the protein.
