Bio-abio interfaces
Fibril structural polymorphism and formation mechanisms
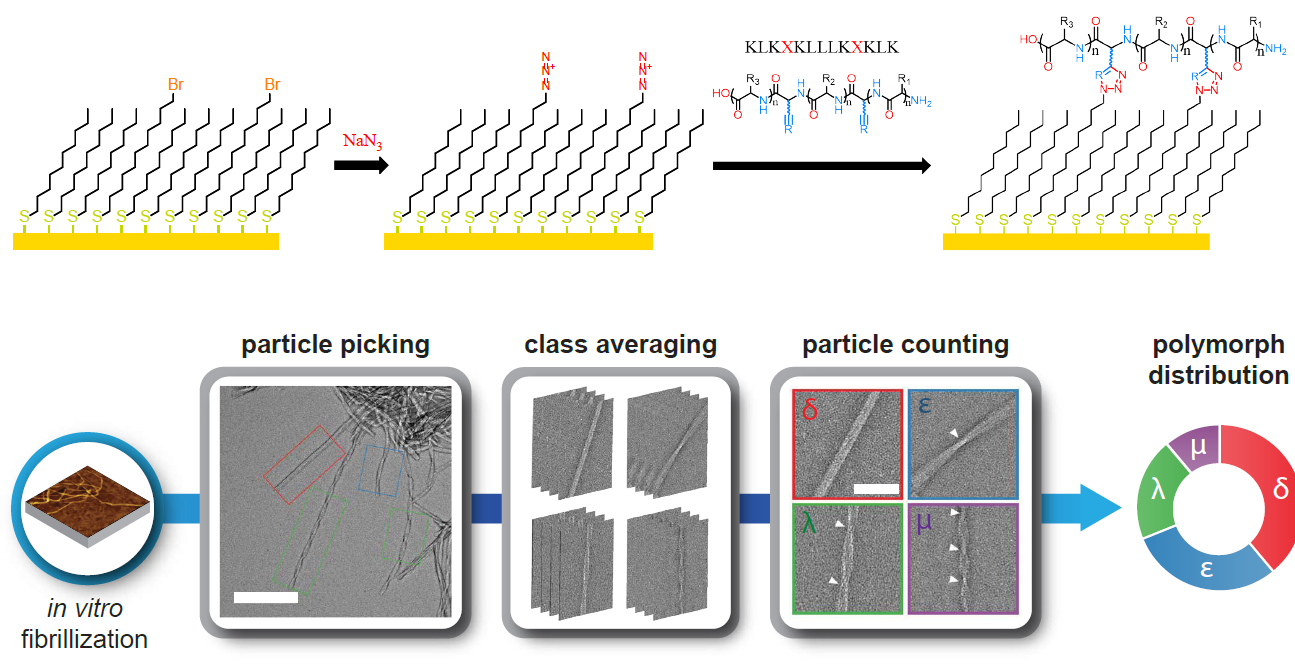
Amyloidogenic proteins have been implicated in an increasing number of neurodegenerative diseases, such as Alzheimer’s disease and type II diabetes. The fibrils that these proteins produce can yield different structures or polymorphs depending on nucleation conditions, which can impact structure-function relationships and disease pathology. We are interested in understanding the impact that nucleation conditions have on fibril polymorphism and bridging the gap between fibrils formed in vitro and in vivo through a variety of approaches. We use chemically tunable self-assembled monolayers (SAMs) as biomimetic surface model systems for amyloid fibrillization by covalently binding peptides to the SAM surfaces, and then characterizing fibril structures with a wide variety of surface characterization techniques. We are then interested in assessing the resulting cytotoxicity of these fibrils in in vivo studies.
Biofunctionalization of gold nanoparticles
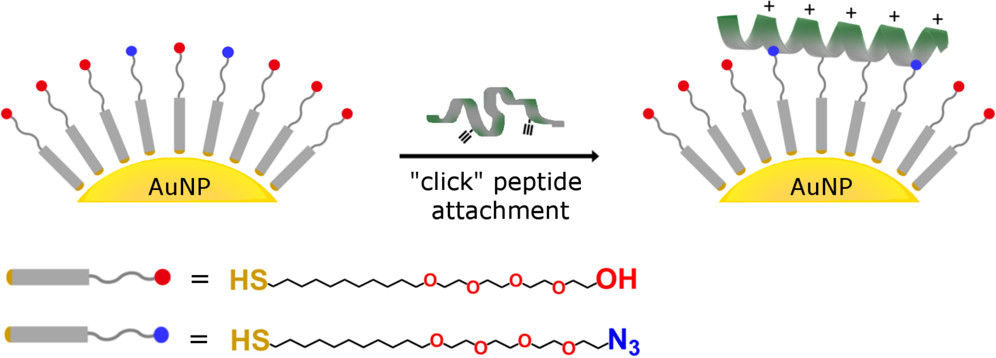
Nanoparticles have optical and electronic properties that are useful for a variety of applications, ranging from sensing to electrochemistry to bio-catalysis. They are particularly useful as they can be immobilized on a multitude of different surfaces from plastics to metal electrodes and have the potential to be used in drug delivery. As such, in collaboration with the Crooks Group, we are looking to expand our peptide functionalization strategies from planar, macroscale gold surfaces to nanoparticles. Our goal is to control protein immobilization onto nanoparticles by exploiting electrostatic interactions between covalently clicked peptides and proteins of interest.
Peptide functionalized surfaces for protein immobilization
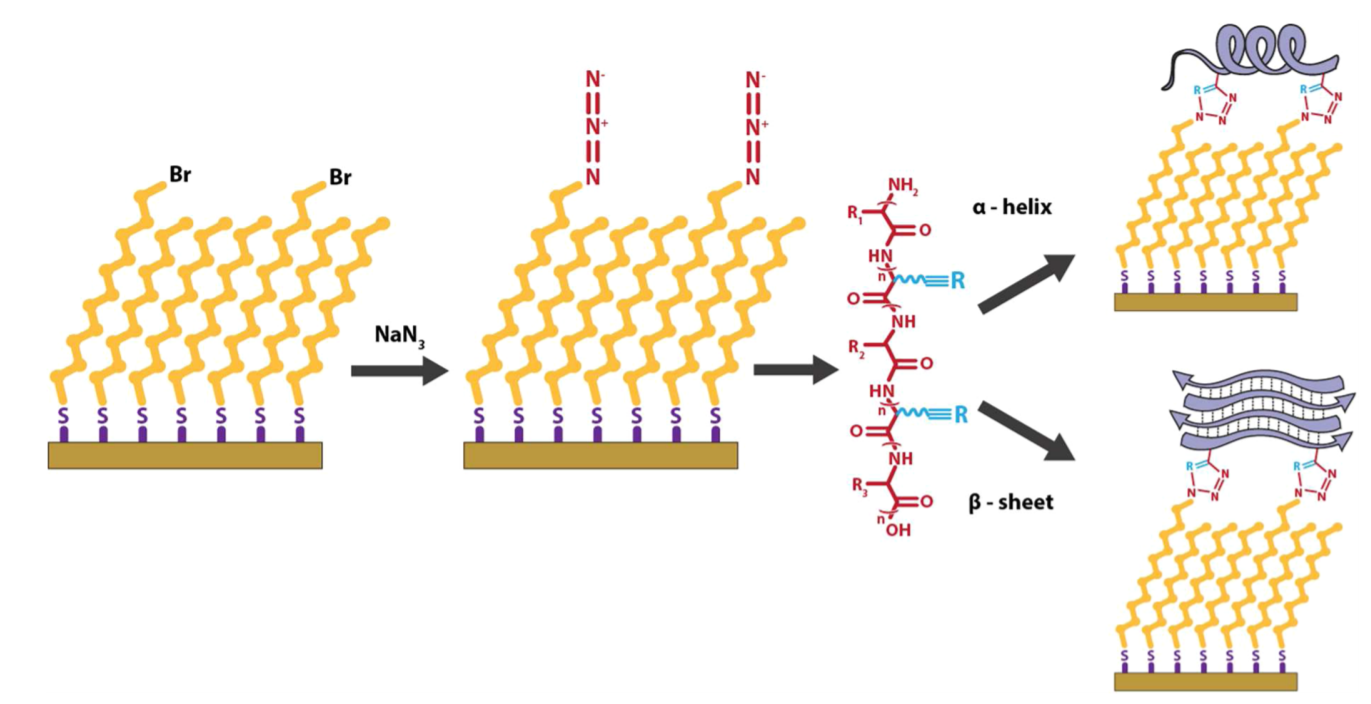
We have developed a method of functionalizing abiotic surfaces with peptides of controlled secondary structure to be used as biomimetic platforms for protein immobilization. To do this, we form self-assembled monolayers (SAMs) of alkyl thiols on gold with reactive functional groups exposed to solution. These functional groups act as reactive sites that can be linked to a specific reactive side chain on a peptide of our choice, thus binding the peptide to the surface. The secondary structure of the peptide is controlled by the peptide sequence and the chemical properties of the SAM / liquid interface. We use techniques such as infrared spectroscopy, circular dichroism spectroscopy, and molecular dynamics simulations (see MD page for more details) to study the secondary structure of the peptide on the surface. Historically, we have used copper-catalyzed azide-alkyne click chemistry to bind peptides to SAMs. Currently, we are looking to expand our arsenal of binding chemistries.
Controlling protein absorption on biomimetic surfaces
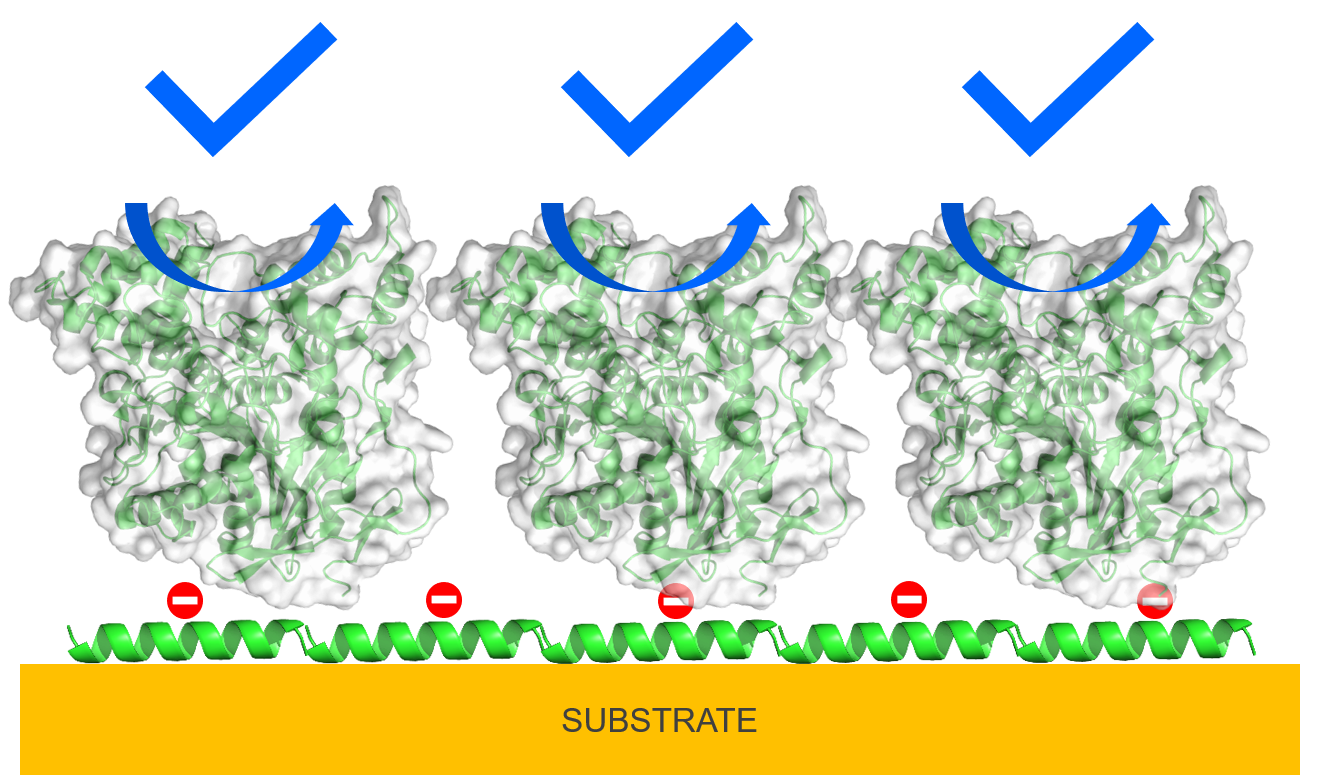
Controlling the interactions between functional enzymes and abiotic substrates is critical in developing enzyme-based devices, such as sensors and fuel cells. Uncontrolled interactions can lead to loss of enzyme function upon immobilization through unfolding and blocking of ligand access to active sites by the substrate and neighboring enzyme molecules, ultimately hindering device performance. Our goal is to chemically functionalize substrates with a monolayer of peptides to block the direct interactions with the substrate, retain enzyme structure, and direct optimal enzyme orientation for ligand access through electrostatic interactions leading to preservation of enzyme function following immobilization.
