Studying electrostatic fields at protein-protein interfaces
Measuring the electrostatics of the the Arf-GEF-BFA interface
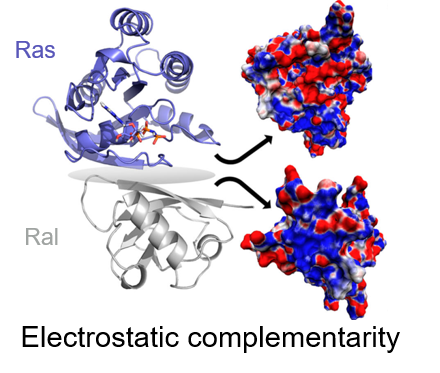
Macromolecular interactions are essential for most cellular processes and being able to drug and regulate these interactions would transform healthcare. However, design of interfacial inhibitors, drugs that target the interface of molecular complexes, has proven incredibly difficult because the surfaces of these interactions are often unobvious, flat binding sites, where both structural and electrostatic complementarity dominate specificity. The structural aspects of many of these interactions are well-studied, but the electrostatic forces, the attractive or repulsive forces between charges and/or partial charges, at these interfaces are rarely understood and quantified despite being crucial for drug design. Computational approaches would be the ideal route for quantifying electrostatic interactions in complexes, but most computational approaches are currently unreliable for calculating electric field and need experimental results for refinement and validation. We are studying the electrostatic interactions in a variety of systems using vibrational stark effect (VSE) spectroscopy (see below), the impact of electrostatic changes on complex formation rates using kinetics, and the structural changes of the complexes using molecular dynamics simulations (see MD page for more details).
References:
Ritchie, A. W.; Webb, L. J., Understanding and Manipulating Electrostatic Fields at the Protein–Protein Interface Using Vibrational Spectroscopy and Continuum Electrostatics Calculations. The Journal of Physical Chemistry B 2015, 119 (44), 13945-13957.
Ragain, C. M.; Newberry, R. W.; Ritchie, A. W.; Webb, L. J., Role of Electrostatics in Differential Binding of RalGDS to Rap Mutations E30D and K31E Investigated by Vibrational Spectroscopy of Thiocyanate Probes. The Journal of Physical Chemistry B 2012, 116 (31), 9326-9336.
Mutations in Ras superfamily of proteins
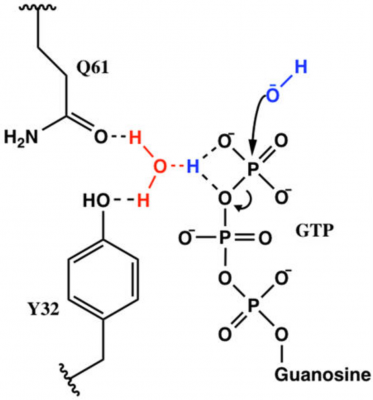
Ras is a GTPase involved in regulating proliferation of cells, and mutations to Ras can be carcinogenic. Carcinogenic mutations are often related to a change in the GTP hydrolysis rate of each Ras mutant. Understanding how each mutation effects GTP hydrolysis of Ras is crucial in understanding how to drug these carcinogenic mutants. Q61 is one of such mutations, and our group found that while Q61 is not directly involved in hydrolysis of GTP, it instead stabilizes the electrostatic organization of the transition state. It appears that Y32 is also involved in this transfer, but, unlike Q61, mutants of Y32 are not often seen in cancer. Our lab is continuing this research by testing the effects of Y32X mutations on the electrostatic environment using vibrational stark effect spectroscopy, the rate of GTP hydrolysis using kinetics experiments, and the structure and solvent accessibility of Y32X-Ras using molecular dynamics simulations.
References:
Novelli, E. T.; First, J. T.; Webb, L. J., Quantitative Measurement of Intrinsic GTP Hydrolysis for Carcinogenic Glutamine 61 Mutants in H-Ras. Biochemistry 2018, 57 (44), 6356-6366.
Vibrational Stark Effect
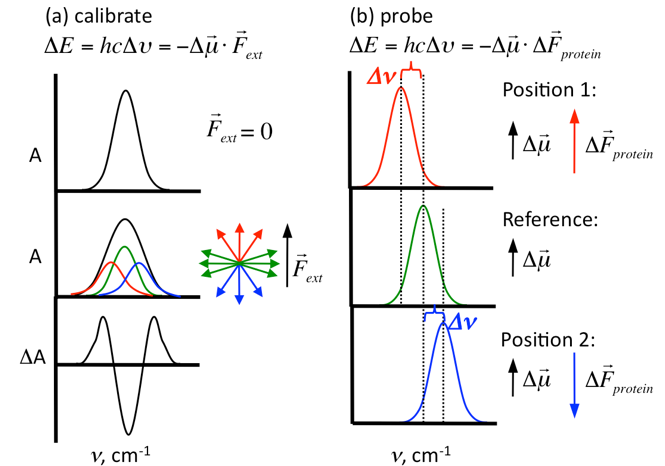
Vibrational Stark effect (VSE) spectroscopy is used in our laboratory to determine changes in electric field in proteins or other biomolecules. We calculate the change in electric field (∆F protein) of a specific bond by measuring changes in the mean vibrational frequency (∆n) of a harmonic oscillator as a function of the magnitude and direction of the local electric field. Incorporating these probes into proteins allows us to measure changes in electric field by comparing proteins in a complex vs. proteins free in solution, protein complexes with and without drug, wildtype proteins vs. mutant proteins, etc. in order to determine the effects of each change on the electric field around these probes.
protein) of a specific bond by measuring changes in the mean vibrational frequency (∆n) of a harmonic oscillator as a function of the magnitude and direction of the local electric field. Incorporating these probes into proteins allows us to measure changes in electric field by comparing proteins in a complex vs. proteins free in solution, protein complexes with and without drug, wildtype proteins vs. mutant proteins, etc. in order to determine the effects of each change on the electric field around these probes.
