Improper Rotations Sn
An improper rotation may be thought of as two steps taken in either order. A rotation and a reflection in a plane, perpendicular to the rotation axis. This axis is referred to as an axis of improper rotation (or an improper axis) and has the symbol Sn where n denotes the order. Obviously, if an axis Cn and a perpendicular plane exist independently in a molecule then Sn exists also. However, Sn may exist when neither the Cn nor the perpendicular σ exist separately. A classic example is ethane C2H6 in the staggered configuration. This molecule has an S6 axis which is coincident with a C3 axis (running along the C-C bond). The combination of rotation and reflection always gives the same result regardless of the order in which they are performed.
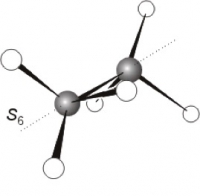
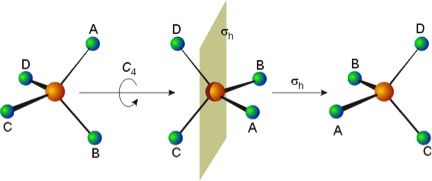
Another example is that of a regular tetrahedral molecule. For example: methane (CH4) has three C2 axes, each of which is simultaneously an S4 axis.
Next: Symmetry Point Groups
Copyright © 2015 Richard Jones. All Rights Reserved.

