Reflections σ and Mirror Planes
A plane of reflection in a molecule can be viewed as a double-sided mirror which bisects the molecule. It must pass through the molecule and cannot be completely outside it. The water molecule has two mirror planes, one is shown below.
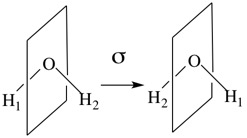
This plane contains the C2 axis and passes between the two H atoms. The effect of this σ symmetry operation is to exchange H1 and H2. The other mirror plane is the one which contains all three atoms of H2O and is perpendicular to the first mirror plane show above. For this plane, H1 and H2 do not exchange positions. It is, nevertheless, a valid symmetry element and symmetry operation for the H2O molecule.
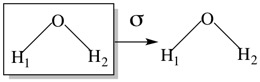
The two planes of symmetry both contain the principle axis (C2), which is designated as the vertical axis and are therefore denoted σv. In order to distinguish the two planes σv and σv| are often used where σv| contains all the atoms.
Note - the symmetry operation σ followed by another σ will result in the molecule returning to its original configuration. In other words, σ2 = E
Next: Inversions
Copyright © 2015 Richard Jones. All Rights Reserved.

